Natural latex is widely used in household and medical applications, but carcinogenic substances such as N-nitrosamines may be present in latex products such as pacifiers, condoms and medical catheters. Most of the traditional accelerators are secondary amine-type vulcanisation accelerators, which when vulcanised at high temperatures can quickly form carcinogenic N-nitrosamines with air or nitrogen oxides in the compound.
Most countries have strict requirements on the content of N-nitrosamines in latex products, and researchers have been working to reduce the content of N-nitrosamines in latex products. There have been studies on the use of primary amine accelerators such as TBSI and TBBS instead of secondary amine accelerator NOBS; the use of accelerators with high relative molecular mass, high melting point and difficult decomposition instead of accelerator TMTD; the use of accelerators with high spatial resistance and less likely to react to produce N-nitrosamines, ZDiBC instead of accelerator ZDC; the use of nitrosamine inhibitors and eliminators, the use of non-amines The accelerators zinc dithiophosphate and zinc xanthate are used.
Accelerator ZDTP has excellent resistance to vulcanisation reversion, good compatibility with rubber, not easy to spray frost, mainly used in EPDM rubber and natural rubber (NR) tyre tread rubber. Thiazole accelerator ZMBT does not produce N-nitrosamines during the vulcanisation process and can be used with accelerator ZMBT and accelerator DPG(D) instead of accelerator ZDEC, making N-nitrosamines undetectable in rubber. The application of the environmentally friendly accelerator ZDTP in natural latex has not been reported. Preliminary studies have found that the accelerator ZDTP has a greater effect on the colloidal properties of the blended latex and a smaller effect on the vulcanisation of the blended film under the sulphur yellow vulcanisation system, while the accelerator ZDTP has a synergistic effect with thiazole accelerators, which can improve the adverse effect of the accelerator on the colloidal properties of the latex.
We learned from our experiments that the viscosity of the blended latex with accelerator ZDTP was significantly higher than that of the blended latex with accelerator ZMBT and ZDTP/ZMBT, and with the extension of storage time, the viscosity of the blended latex with accelerator ZDTP increased rapidly, increasing 861. 5% during the storage period. The viscosity of the blended latex made with accelerator ZMBT or ZDTP/ZMBT was small and increased gradually with the increase in the amount of accelerator ZDTP. This is because the solubility of the accelerator ZDTP is greater than that of the accelerator ZMBT, the concentration of zinc ions generated by the ionisation of the accelerator ZDTP is high, and the zinc ions and ammonium salts generated by the zinc ammonia complex ions are easy to react with the advanced fatty acid soap adsorbed on the surface of the rubber particles to generate insoluble zinc soap, which reduces the negative charge on the surface of the rubber particles and dehydrates the rubber particles; at the same time, the concentration of zinc ions increases, increasing the ionic strength of the whey phase. These reduce the stability of the blended latex made with the accelerator ZDTP, thus increasing the viscosity of the latex. As the storage time increases, the ZDTP accelerator gradually dissolves, the zinc-ammonia complex ion concentration increases, the destabilising effect is enhanced and the viscosity of the blended latex increases.
In the system with accelerator ZDTP/ZMBT, the viscosity of the blended latex changes less with the extension of the storage time, and the smaller the amount of accelerator ZDTP, the smaller the change of the viscosity of the blended latex. When the dosage of accelerator ZDTP/ZMBT was 1/0.5, the viscosity of the blended latex did not change much in the early and late stages of storage, while the viscosity of the blended latex increased slightly in the middle stage of storage. The analysis suggests that, on the one hand, zinc oxide and ZDTP dissolved to produce zinc ions and zinc ammonia complex ions, which had a destabilizing effect on the latex; on the other hand, phosphate ions could react with zinc ions and zinc ammonia complex ions to reduce the destabilizing effect. In the early stage of storage, phosphate ions react with zinc ions and zinc ammonia complex ions to reduce the viscosity of the latex; in the middle stage of storage, the reaction of phosphate ions is basically completed, while zinc oxide and accelerator ZDTP continue to dissolve, zinc ions and zinc ammonia complex ions continue to be generated, so the viscosity of the latex increases; in the late stage of storage, zinc oxide and accelerator ZDTP dissolve to equilibrium, and the concentration of zinc ions and zinc ammonia complex ions no longer The viscosity of the blended latex stabilises.
The thermal stability of the latex made with the accelerator ZDTP is low and gradually decreases as the storage time is extended; the thermal stability of the latex made with the accelerator ZDTP/ZMBT is improved and gradually decreases as the amount of the accelerator ZDTP increases. This is due to the fact that an increase in the amount of accelerator ZDTP increases the concentration of zinc ions and increases the amount of zinc ammonia complex ions produced by the reaction, which is a heat-sensitive agent and therefore reduces the thermal stability of the blended latex.
In the accelerator ZDTP/ZMBT mixture system, when the accelerator ZDTP dosage is larger, such as the accelerator ZDTP/ZMBT dosage ratio is 1:2.5, the thermal stability of the latex with the extension of storage time and slightly decreased, this is because the accelerator ZDTP dosage is larger, the zinc ammonia complex ion concentration is large, phosphate and zinc ammonia complex ion in the early stage of storage almost completely reaction, in the storage process The process does not change much. When the accelerator ZMBT is used alone, the solubility of ZMBT is small and the zinc ions mainly come from the dissolved zinc oxide. The low thermal stability of the latex in the early stages of storage is due to the greater destabilising effect of the zinc-ammonia complexes generated by the dissolution of zinc oxide, and the increased thermal stability of the latex in the later stages of storage is due to the fact that the dissolution of zinc oxide is basically complete and the presence of phosphate can improve the thermal stability of the latex.
The mechanical stability of the latex made with the rubber accelerators ZDTP/ZMBT is significantly greater than that of the blended latex made with the accelerators ZDTP or ZMBT alone, and the mechanical stability of the blended latex made with the accelerator ZMBT is the smallest. This is because the phosphate produced by the ionisation of ammonium dihydrogen phosphate can react with zinc ammonia complex ions to produce insoluble zinc salts, which reduces the destabilising effect of zinc ammonia complex ions and improves the mechanical stability of the latex, but when the amount used is too large, the ionic strength of the whey phase is greater, resulting in reduced mechanical stability. This is because the solubility of the accelerator ZDTP is larger than that of the accelerator ZMBT, with the high concentration of zinc ions and zinc ammonia complex ions in the latex, zinc ammonia complex ions can not completely react with phosphate, so the mechanical stability of the latex with the accelerator ZMBT, the amount of ammonium dihydrogen phosphate far exceeds the equivalent amount of zinc ammonia complex ions, the whey phase ion concentration increases, so the mechanical stability of the latex with the accelerator ZMBT is also smaller.
When the dosage ratio of ZDTP/ZMBT was 0.75:0.75, the mechanical stability of the latex was greater because the phosphate could react with the zinc-ammonia complex ion more fully without increasing the ionic strength of the whey phase; when the dosage ratio of ZDTP/ZMBT was 0.5:1, the mechanical stability of the latex first increased and then decreased rapidly, and finally levelled off. The analysis suggests that in the early stage of storage, the zinc-ammonia complex ion generated by the dissolution of zinc oxide and accelerator ZDTP reacts with phosphate, and the mechanical stability of the blended latex increases, when the dissolution of zinc oxide and accelerator ZDTP is basically completed, the zinc-ammonia complex ion is no longer generated, and the excessive phosphate reduces the mechanical stability of the blended latex. When the dosage of accelerator ZDTP/ZMBT is 0.5/1, the mechanical stability of the blended latex decreases significantly than when the dosage of accelerator ZDTP/ZMBT is 0.75/0.75. This is because the remaining ammonium dihydrogen phosphate in the former is more than in the latter, and the ionic strength of the whey phase increases significantly than in the latter. When the accelerator ZDTP/ZMBT dosage was 1:0.5, the mechanical stability of the blended latex varied with storage time for similar reasons to the accelerator ZDTP/ZMBT dosage ratio of 0.75/0.75.
Tables below: Vulcanisation characteristics of blended latex films made with different accelerators.

It can be seen from the table: when accelerator ZDTP and ZMBT are used alone, the vulcanization rate of the film is slow and the degree of cross-linking is low; when accelerator ZDTP/ZMBT is used, the vulcanization rate of the film is obviously accelerated and the degree of cross-linking is greatly improved; when the dosage ratio of accelerator ZDTP/ZMBT is 1/0.5, the vulcanization rate of the film is the fastest; the degree of cross-linking varies little with the dosage ratio of accelerator ZDTP/ZMBT. The ZMBT dosage ratio did not change much. It can be seen that the use of accelerator ZDTP and ZMBT has a mutual activation and activation effect, and has a good synergistic effect so that the film can obtain a faster vulcanization speed and a higher degree of crosslinking.
Physical properties of vulcanised films. The physical properties of the vulcanised films produced with different accelerators are shown in the table.
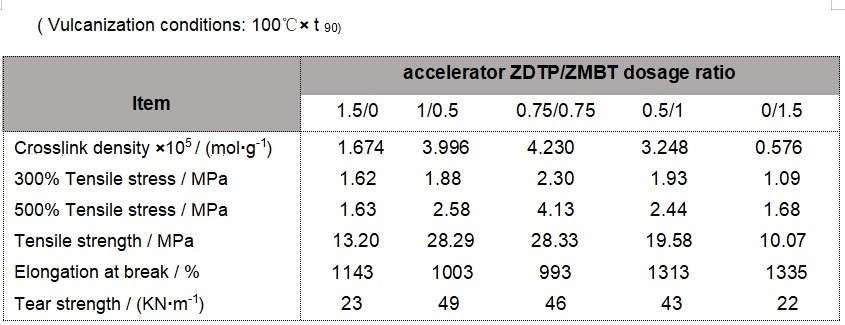
As can be seen from the table, the crosslink density, tensile stress, tensile strength and tear strength of the vulcanized rubber film are small when accelerator ZDTP and ZMBT are used alone; accelerator ZDTP/ZMBT with synergistic effect can increase the crosslink density of the vulcanized rubber film and improve the physical properties of the vulcanized rubber film.
In the ZDTP/ZMBT system, as the dosage of ZDTP decreases, the crosslink density, tensile stress and tensile strength of the vulcanized film increase and then decrease, the elongation at break decreases and then increases, and the tear strength gradually decreases. When the dosage of accelerator ZDTP/ZMBT was 0.75:0.75, the crosslink density, tensile stress and tensile strength of the vulcanized film were the largest and the elongation at break was the smallest. When the dosage of accelerator ZDTP/ZMBT was 1:0.5, the tear strength of the vulcanized film was the largest. The analysis shows that when the vulcanization rate is too large, the cross-linking reaction occurs unevenly and the cross-linked rubber molecules restrict the movement of the uncross-linked rubber molecules, which leads to a low degree of cross-linking, uneven network structure and reduced physical properties. It can be seen that when the dosage ratio of accelerator ZDTP/ZMBT is 0.75:0.75, the vulcanisation rate of the rubber film is faster, and the crosslinking density and comprehensive physical properties are better. Therefore, the use of accelerator ZDTP and ZMBT can solve the problems of poor colloidal stability, low crosslinking density and poor physical properties of the latex made from accelerator ZDTP.
Conclusion
(1) The viscosity of the blended latex made with accelerator ZDTP is large, and the thermal stability and mechanical stability are small; the use of accelerator ZDTP/ZMBT can improve the performance of the blended latex colloid, with the increase of the dosage of accelerator ZDTP, the viscosity of the blended latex gradually increases, the thermal stability gradually decreases, and the mechanical stability reaches a better value when the dosage ratio is 0.75:0.75.
(2) The accelerator ZDTP/ZMBT has a good synergistic effect, the vulcanization rate of the film is accelerated and the degree of cross-linking is increased; the vulcanization rate of the film is fastest when the dosage ratio of accelerator ZDTP/ZMBT is 1:0.5; the cross-linking density of the film is greatest when the dosage ratio of accelerator ZDTP/ZMBT is 0.75:0.75, and the comprehensive physical properties are better. The overall physical properties were better.

